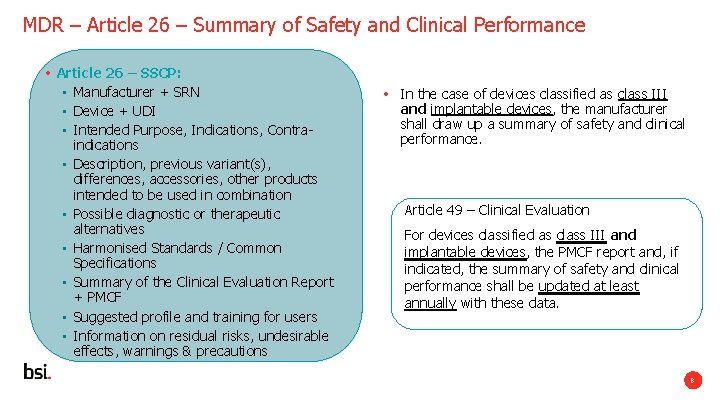
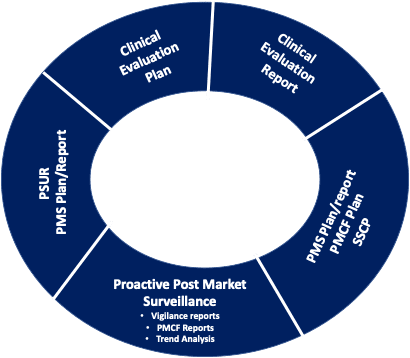
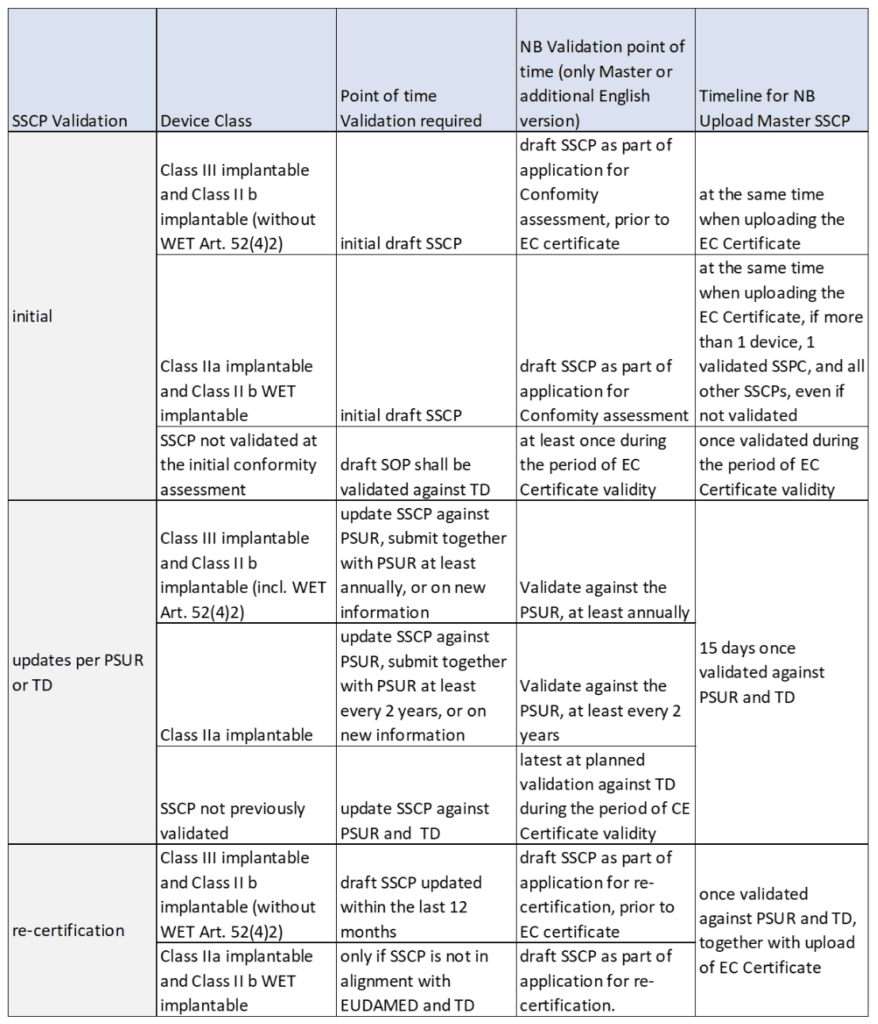
The Summary of Safety and Clinical Performance (SSCP) is a Required Part of Medical Device Regulation (MDR) 2017/745: Is Your Organization Ready? - Criterion Edge

Medical Devices Clinical Evaluation - Summary of Safety and Clinical Performance (SSCP) - Regulation (EU) 2017/745 - GMED Medical Device Certification
Bicon SynthoGraft Summary of Safety and Clinical Performance Document number: SSCP-002 Document revision: 01 Date issued: April

Summary of Safety and Clinical Performance (SSCP): 5 Challenges to be met - GMED Medical Device Certification


![Summary of Safety and Clinical Performance [ISO 13485 templates] Summary of Safety and Clinical Performance [ISO 13485 templates]](https://advisera.com/wp-content/uploads//sites/14/2021/08/24.3_Summary_of_Safety_and_Clinical_Performance_Integrated_Preview_EN.png)