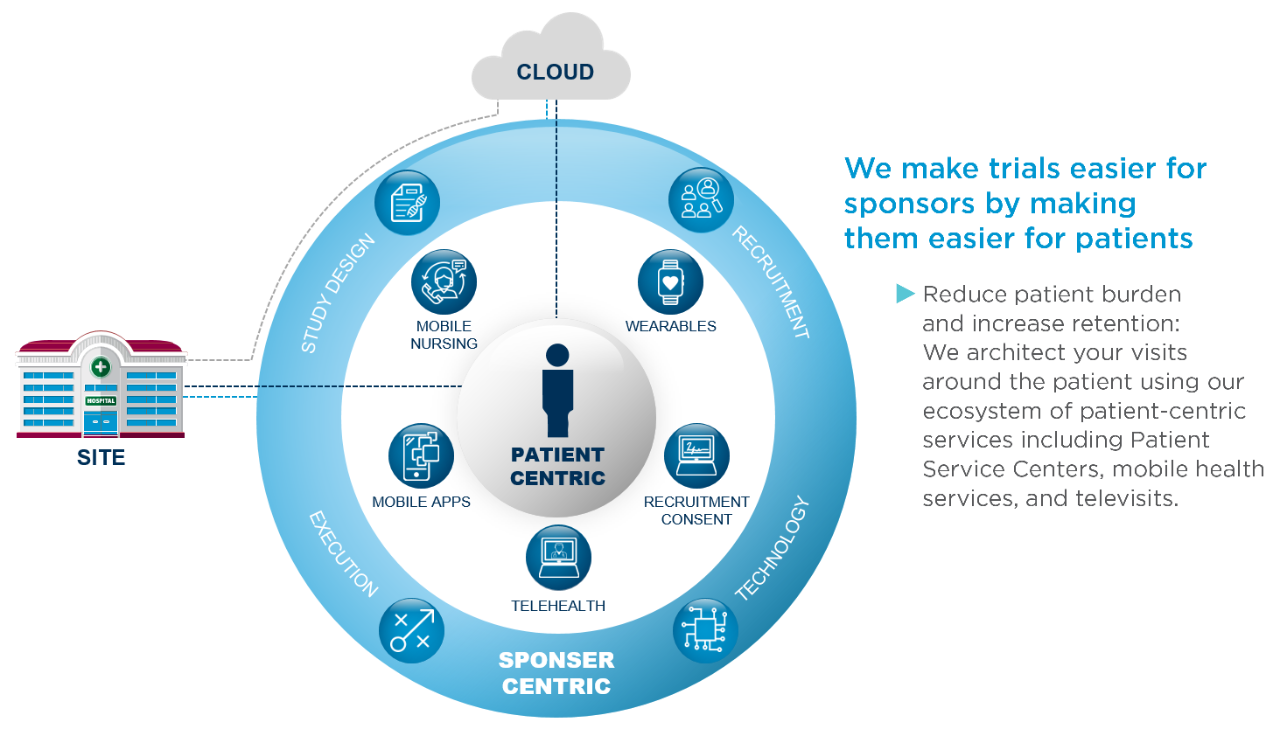
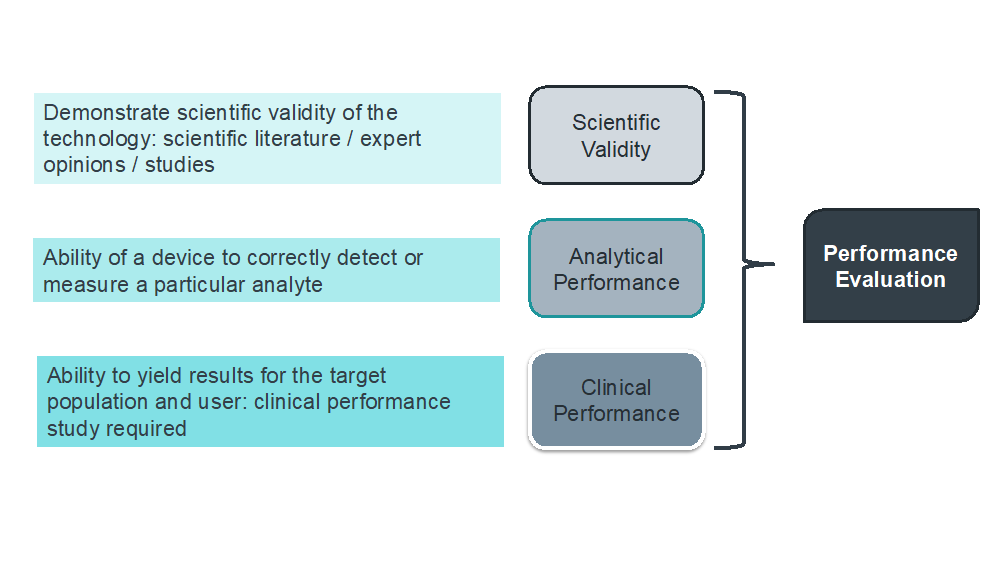
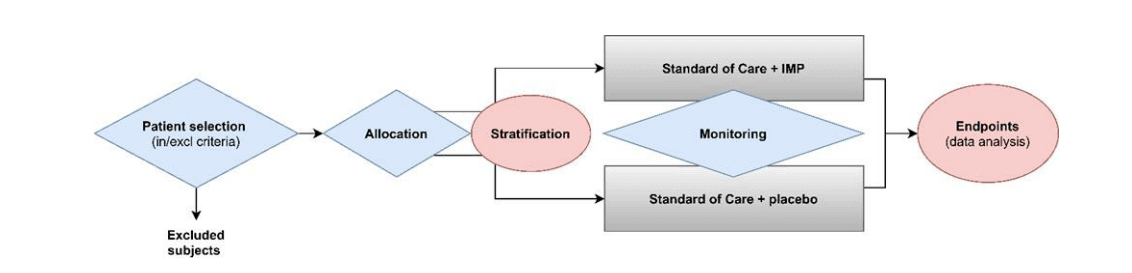
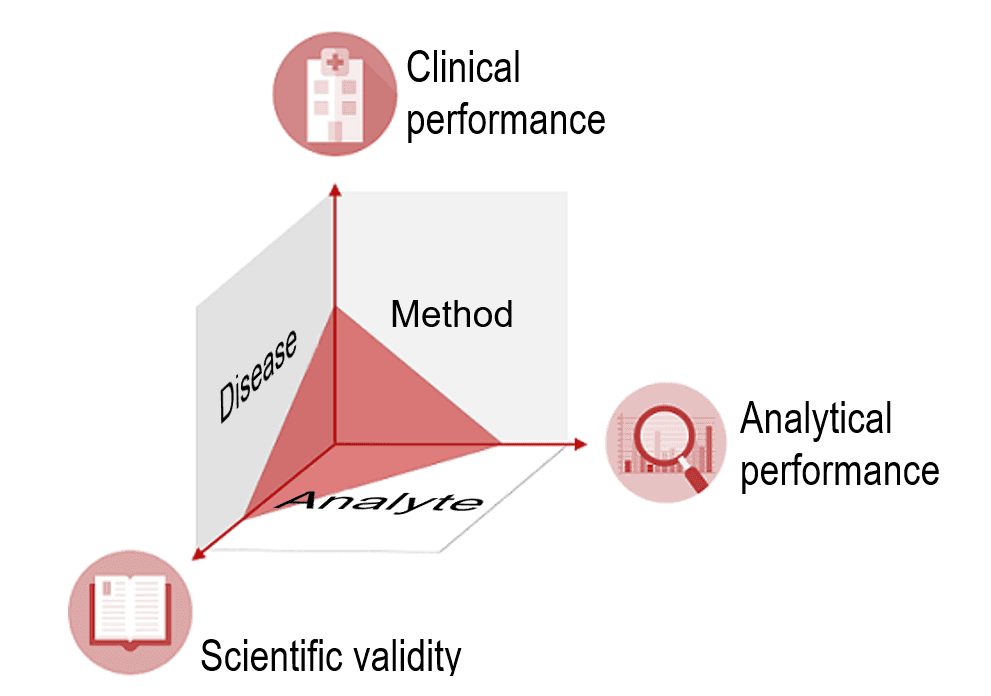
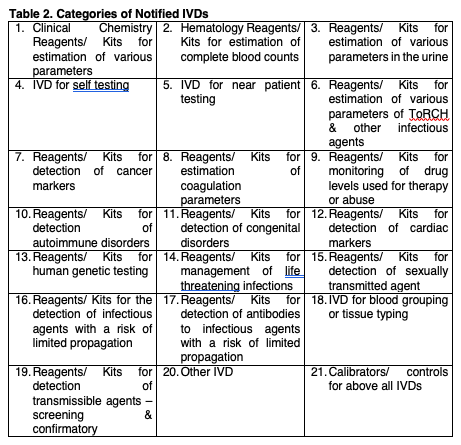
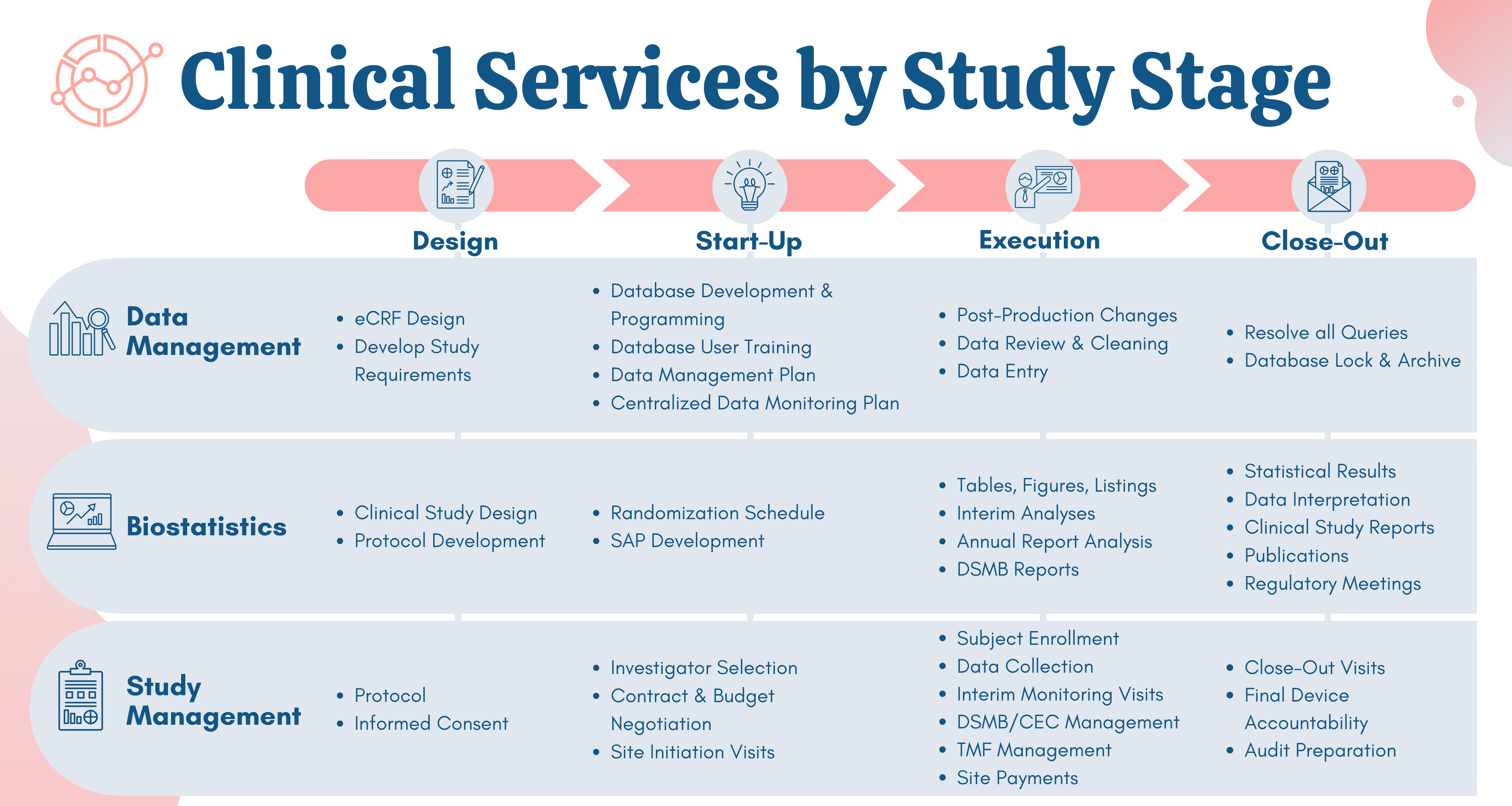
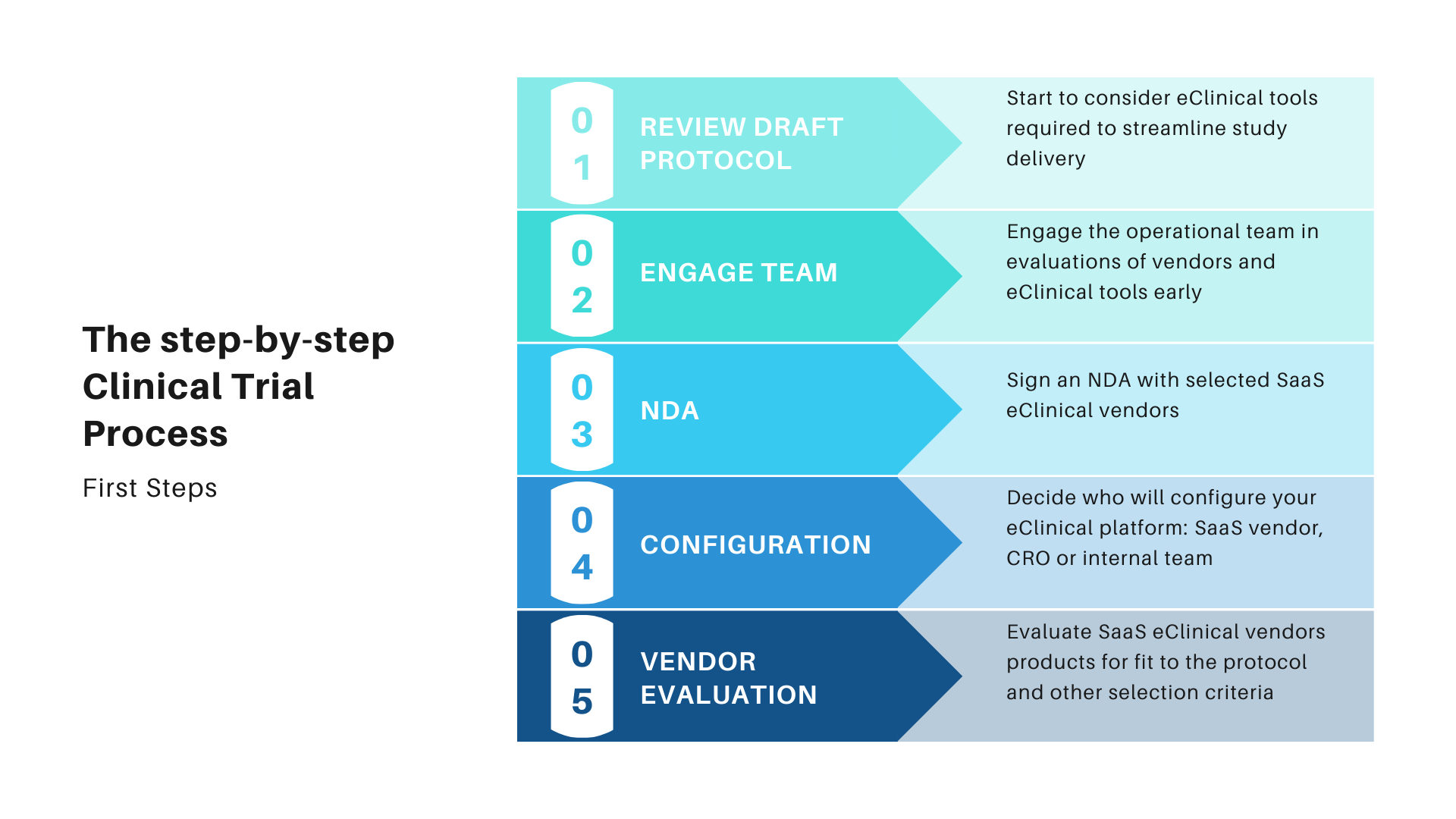
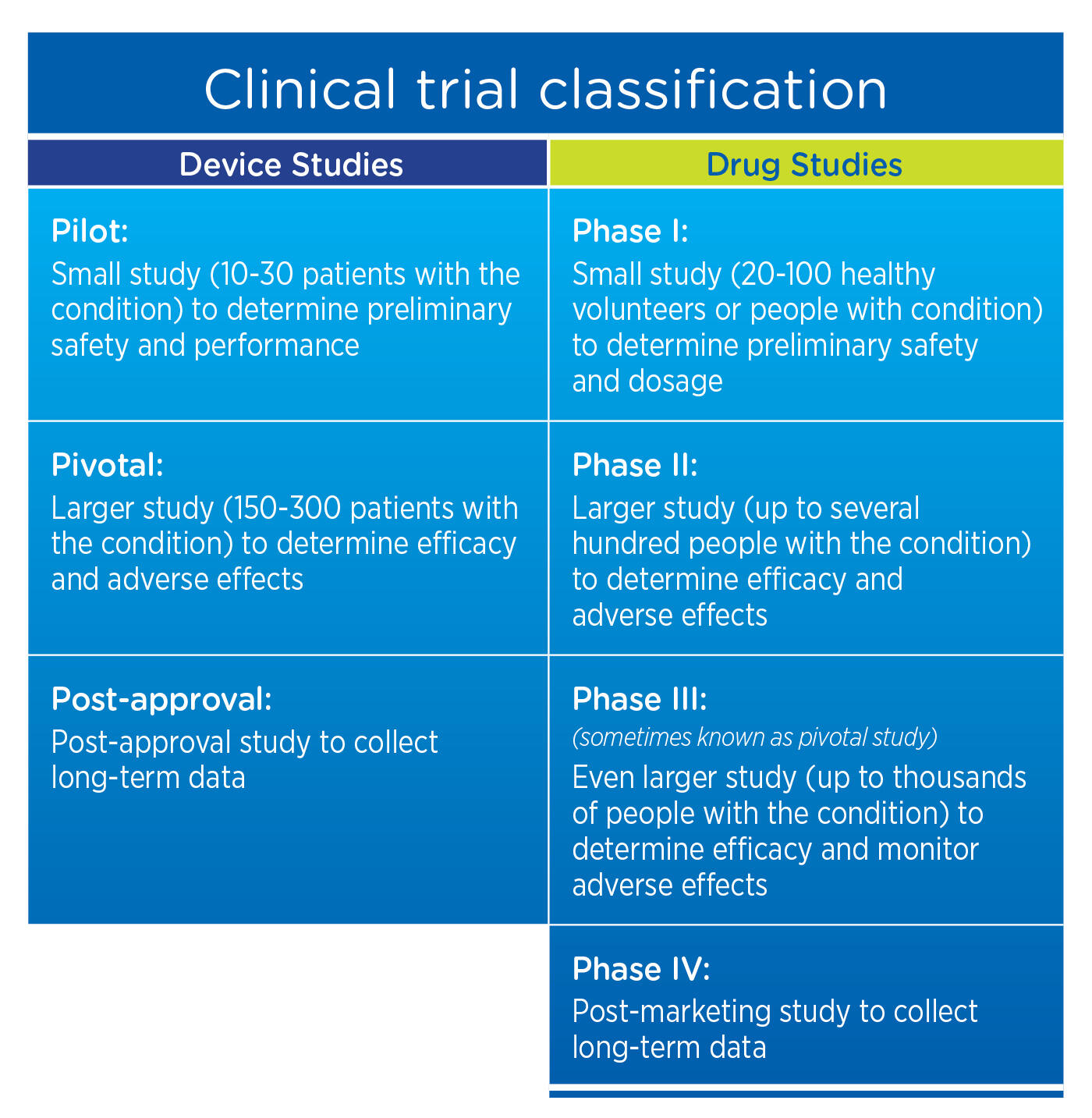
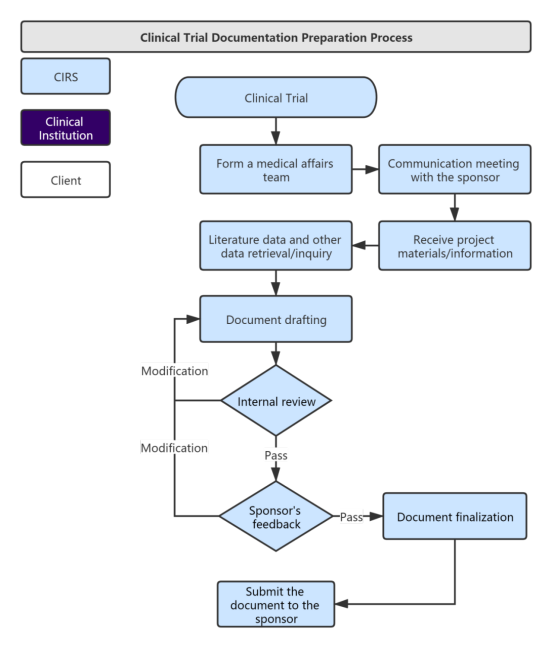
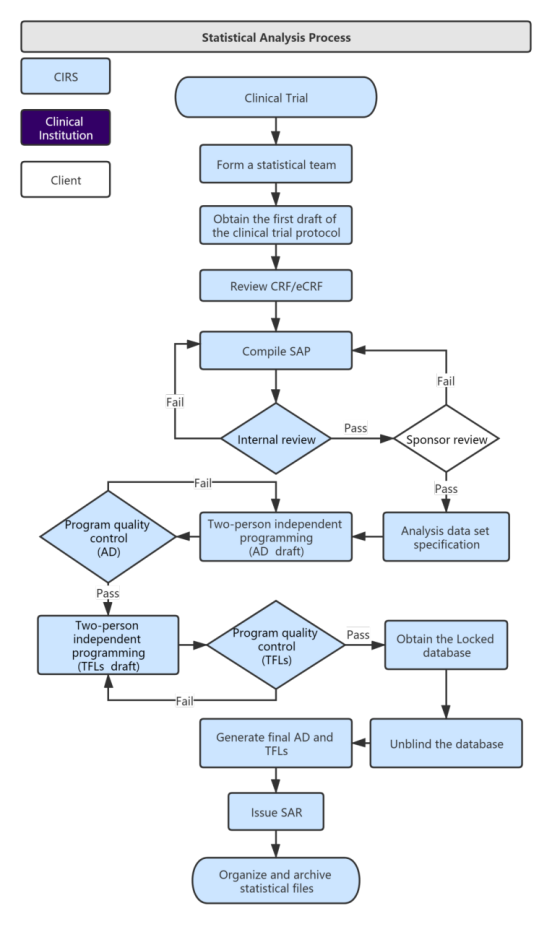
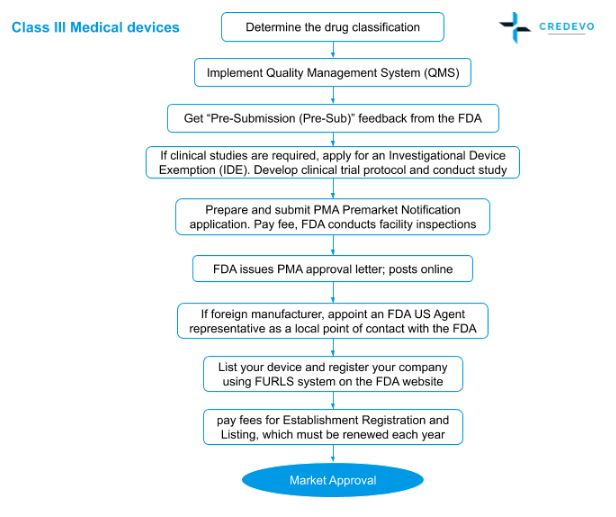
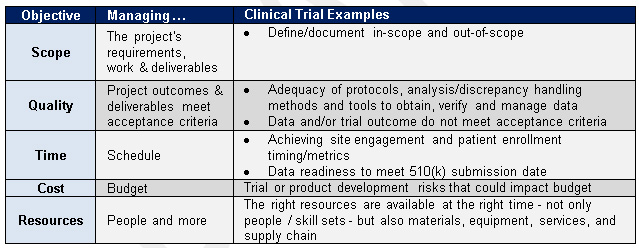
Overview of the process of in vitro diagnostic (IVD) test development... | Download Scientific Diagram
MDCG 2022-2 Guidance on general principles of clinical evidence for In Vitro Diagnostic medical devices (IVDs)

In Vitro Diagnostic (IVD) Development | Clinical Research Associate CRA - Career, Jobs, Certification, Industry Insight.