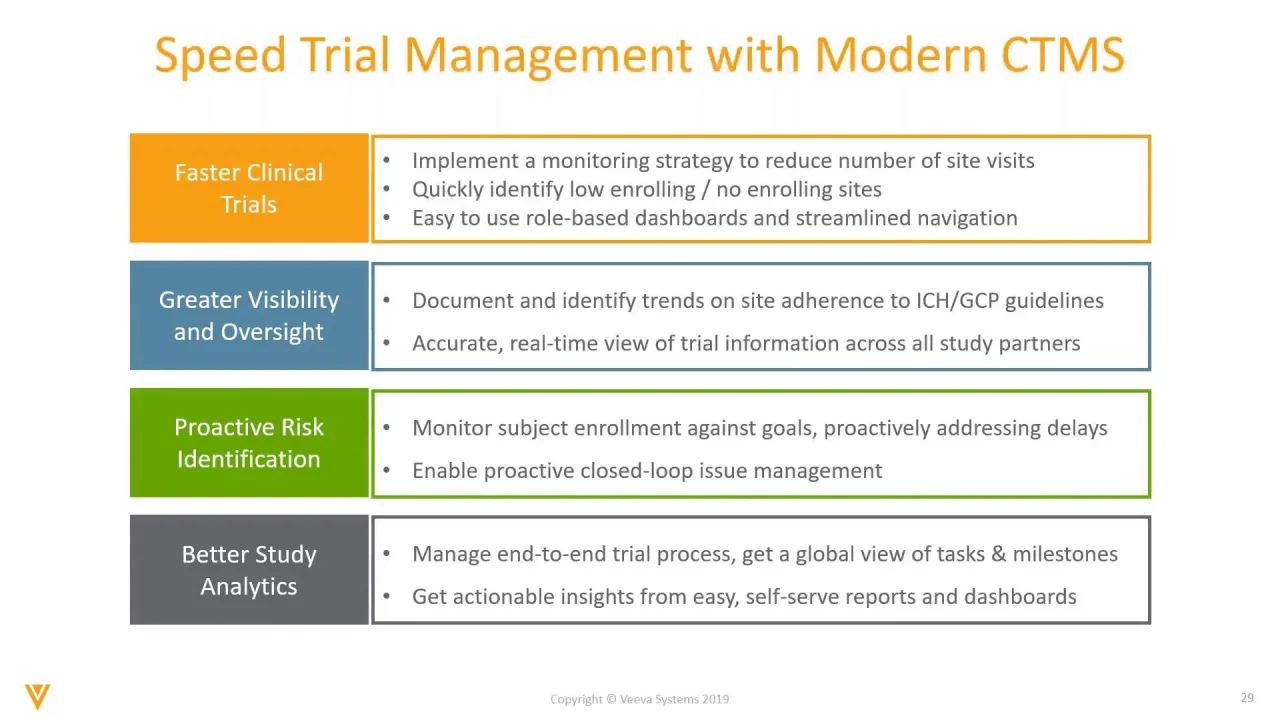
ICTD 2021 - EMA Clinical Trial Information System (CTIS) for platform trials - Noémie Manent - YouTube

CTIS – M07 How to create a CT: Clinical Trial centric approach vs organisation centric approach - YouTube

EU Medicines Agency on Twitter: "Clinical trial sponsors can now consult a compilation of key guidance, technical information, recommendations and references that will help them to get ready for the use of